What vaccine for COVID-19 is currently available?
The Pfizer/BioNTech COVID-19 vaccine is available. This vaccine is shown to offer up to 95% efficacy and has been given regulatory approval by the MHRA.
The Government has in principle secured access to seven different vaccine candidates, across four different vaccine types, totalling over 357 million doses. This includes:
- 40 million doses of the BioNTech/Pfizer vaccine
- 100m doses of the Oxford/AstraZeneca vaccine currently being assessed by the MHRA.
- 7 million doses of the Moderna vaccine, which is also being assessed by the MHRA
Is the NHS confident the vaccine is safe?
Yes. The NHS will not offer any Covid-19 vaccinations to the public until independent experts have signed off that it is safe to do so. The MHRA, the official UK regulator, have said this vaccine is very safe and highly effective, and we have full confidence in their expert judgement and processes.
As with any medicine, vaccines are highly regulated products. There are checks at every stage in the development and manufacturing process, and continued monitoring once it has been authorised and is being used in the wider population.
Will the vaccine work with the new strain?
There is no evidence currently that the new strain will be resistant to the vaccine we have, so we are continuing to vaccinate people as normal. Scientists are looking now in detail at the characteristics of the virus in relation to the vaccine. Viruses, such as the winter flu virus, often branch into different strains but these small variations rarely render vaccines ineffective.
Why are BAME groups not being prioritised?
There is clear evidence that certain Black, Asian and minority ethnic (BAME) groups have higher rates of infection, and higher rates of serious disease, morbidity and mortality.
There is no strong evidence that ethnicity by itself (or genetics) is the sole explanation for observed differences in rates of severe illness and deaths. Certain health conditions are associated with increased risk of serious disease, and these health conditions are often overrepresented in certain Black, Asian and minority ethnic groups.
Societal factors, such as occupation, household size, deprivation, and access to healthcare can increase susceptibility to COVID-19 and worsen outcomes following infection.
Prioritisation of persons with underlying health conditions will also provide for greater vaccination of BAME communities who are disproportionately affected by such health conditions.
The advice is for NHS England and NHS Improvement, the Department of Health and Social Care, Public Health England and the devolved administrations to work together to ensure that inequalities are identified and addressed in implementation.
This could be through culturally competent and tailored communications and flexible models of delivery, aimed at ensuring everything possible is done to promote good uptake in Black, Asian and minority ethnic groups and in groups who may experience inequalities in access to, or engagement with, healthcare services. These tailored implementation measures should be applied across all priority groups during the vaccination programme.
Is the vaccine vegan/vegetarian friendly?
Yes, the Pfizer vaccine does not contain any meat derivatives or porcine products.
If, and when, further vaccines are approved we will publish information about known allergens or ingredients that are important for certain faiths, cultures and beliefs.
Who cannot have the vaccine?
The COVID-19 vaccination is not recommended for women who are pregnant.
People who are suffering from a fever-type illness should also postpone having the vaccine until they have recovered.
Can I go back to work after having my vaccine?
Yes, you should be able to work as long as you feel well. If your arm is particularly sore, you may find heavy lifting difficult. If you feel unwell or very tired you should rest and avoid operating machinery or driving.
The vaccine cannot give you COVID-19 infection, and two doses will reduce your chance of becoming seriously ill. However, you will need to continue to follow the guidance in your workplace, including wearing the correct personal protection equipment and taking part in any screening programmes.
How effective is the COVID-19 vaccine? How long does it take to work?
The MHRA have said this vaccine is highly effective, but to get full protection people need to come back for the second dose – this is really important.
Full protection kicks in around a week or two after that second dose, which is why it’s also important that when you do get invited, you act on that and get yourself booked in as soon as possible.
Is the NHS confident the vaccine will be safe?
Yes. The NHS would not offer any COVID-19 vaccinations to the public until it is safe to do so. The MHRA, the official UK regulator authorising licensed use of medicines and vaccines by healthcare professionals, has made this decision, and we have full confidence in their expert judgement and processes.
As with any medicine, vaccines are highly regulated products. There are checks at every stage in the development and manufacturing process.
What is the evidence to show the vaccine is safe for BAME communities?
The phase three study of the Pfizer BioNTech COVID-19 vaccine demonstrated a vaccine efficacy of 95%, with consistent efficacy across age, gender and ethnicity. Overall, among the participants who received the COVID-19 vaccine 82.1% were White, 9.6% were Black or African American, 26.1% were Hispanic/Latino, 4.3% were Asian and 0.7% were Native American/Alaskan.
I’m currently ill with COVID-19, can I get the vaccine?
People currently unwell and experiencing COVID-19 symptoms should not receive the COVID-19 vaccine until they have recovered.
Do people who have already had COVID-19 get vaccinated?
Yes, they should get vaccinated. There is no evidence of any safety concerns from vaccinating individuals with a past history of COVID-19 infection, or with detectable COVID-19 antibody, so people who have had COVID-19 disease (whether confirmed or suspected) can still receive the COVID-19 vaccine when it is their time to do so.
Are there any known or anticipated side effects?
Like all medicines, vaccines can cause side effects. Most of these are mild and short-term, and not everyone gets them. Even if you do have symptoms after the first dose, you still need to have the second dose. You may not be protected until at least seven days after your second dose of the vaccine.
- Very common side effects include:
- Having a painful, heavy feeling and tenderness in the arm where you had your injection. This tends to be worst around 1-2 days after the vaccine
- Feeling tired
- Headache
- General aches, or mild flu-like symptoms
As with all vaccines, appropriate treatment and care will be available in case of a rare anaphylactic event following administration.
How many doses of the vaccine will be required and when?
You are required to have two doses of the COVID-19 vaccine. You may not be fully protected until at least seven days after your second dose of vaccine.
How long will the Pfizer vaccine be effective for?
We expect these vaccines to work for at least a year – if not longer. This will be constantly monitored.
I have had my flu vaccine, do I need the COVID-19 vaccine as well?
The flu vaccine does not protect you from COVID-19. If you are eligible for both vaccines, you should have them both, but normally separated by at least a week.
Will the COVID-19 vaccine protect me from flu?
No, the COVID-19 vaccine will not protect you against the flu. If you have been offered a flu vaccine, please try to have this as soon as possible to help protect you, your family and patients from flu this winter.
I haven’t had my flu vaccine – can I still have the Covid vaccine?
You can, but we recommend that you have both vaccines as both are dangerous infections. You cannot have the flu and Covid-19 vaccines within 7 days of each other.
Can people pick what vaccine they want?
Any vaccines that the NHS will provide will have been approved because they pass the MHRA’s tests on safety and efficacy, so people should be assured that whatever vaccine they get, it is worth their while.
I have already had Covid-19. Why should I be vaccinated?
We know that immunity after infection is not long lasting in most cases. It is possible to get Covid-19 twice, and the vaccine provides much better immunity than having had the infection as far as we can tell at this stage.
Can I have the vaccine if I am pregnant or breast-feeding?
The vaccine is not recommended if you are pregnant, breastfeeding, or intending to try to conceive within 2 months, so it will not be prescribed in these cases.
I have heard in the media about allergic reactions – should I be worried?
All medications and vaccines have a small risk of minor or more severe allergic reactions. If you have had a severe allergic reaction to anything before, you are unlikely to be permitted to have the vaccine at the moment. It’s very rare for anyone to have a serious reaction to the vaccine (anaphylaxis). If this does happen, it usually happens within minutes. Staff giving the vaccine are trained to deal with allergic reactions and treat them immediately.
Can any member of the public be vaccinated? Can they just go to a mass vaccination site?
People will be offered vaccinations in line with recommendations from the independent JCVI. The NHS will contact people when it is their turn. People will need an appointment to get their vaccine; most people will be invited by letter from their GP practice or the national programme. Please do not turn up at the hospital or any other vaccination sites without an appointment as we will have to turn you away.
How does the vaccine work?
The vaccine works by making a protein from the virus that is important for creating protection.
The protein works in the same way they do for other vaccines by stimulating the immune system to make antibodies and cells to fight the infection.
How is the COVID-19 vaccine given?
The COVID-19 vaccine is given as an injection into your upper arm. It is given as 2 doses.
When will the 2nd dose be given?
The 2nd dose was previously due to be given 21 days after having the 1st dose, but this has now changed to 12 weeks after the 1st dose.
Why has the timing of the 2nd dose been changed?
The latest evidence suggests the 1st dose of the COVID-19 vaccine provides protection for most people for up to 3 months.
As a result of this evidence, when you can have the 2nd dose has changed. This is also to make sure as many people can have the vaccine as possible.
What has changed to make 12 weeks safe for the dose interval when it wasn’t last week?
Having studied evidence on both the Pfizer/BioNTech and Oxford/AstraZeneca vaccines, The Joint Committee on Vaccination and Immunisation (JCVI) has advised that we should prioritise giving as many people in at-risk groups their first dose, rather than providing two doses in as short a time as possible.
The four UK Chief Medical Officers agree with JCVI that, at this stage of the pandemic, prioritising the first doses of vaccine for as many people as possible on the priority list will protect the greatest number of at risk people overall in the shortest possible time and will have the greatest impact on reducing mortality, severe disease and hospitalisations and in protecting the NHS and equivalent health services. This is because the evidence shows that one dose of either vaccine provides a high level of protection from Covid-19.
For both vaccines, data provided to the Medicines and Healthcare products Regulatory Agency (MHRA) demonstrate that whilst efficacy is optimised when a second dose is administered, both offer considerable protection after a single dose, at least in the short term. For both vaccines, the second dose completes the course and is likely to be important for longer term protection
The NHS across the UK will prioritise giving the first dose of the vaccine to those in the most high-risk groups. Everyone will still receive their second dose and this will be within 12 weeks of their first. The second dose completes the course and is important for longer term protection.
The JCVI’s independent advice is that this approach will maximise the benefits of both vaccines allowing the NHS to help the greatest number of people in the shortest possible time. It will ensure that more at-risk people are able to get meaningful protection from a vaccine in the coming weeks and months, reducing deaths and starting to ease pressure on our NHS.
Is the interval between doses being changed because we don’t have enough vaccine?
No. The decision to update the dosing interval is based on advice from the JCVI and MHRA and is designed to maximise the impact of the programme and save lives.
Pfizer say only 52% efficacy after 1 dose. Surely everyone should have 2nd dose after 3 weeks as planned?
The data indicates that from a fortnight after the first dose both vaccines offer a very high level of protection. Updating the dosing interval is in line with the advice of the JCVI, and is the right thing to do, to maximise the impact of the programme and save lives.
Does one dose of the vaccine offer protection?
The JCVI has recommended that as many people on the JCVI priority list as possible should be offered a first vaccine dose as the initial priority. This is because one dose of the vaccine offers important protection and we want to reach as many at risk people as possible in order to offer protection until the second dose can be administered.
They have advised that the second dose of the Pfizer-BioNTech vaccine may be given between 3 to 12 weeks following the first dose, and that the second dose of the AstraZeneca (Oxford) vaccine may be given between 4 to 12 weeks following the first dose. The clinical risk priority order for deployment of the vaccines remains unchanged and applies to both vaccines. Both are very effective vaccines.
What about people who have already had their 2nd dose after 3 weeks? Is this safe? Will they be protected?
Yes. The updating of the dosing interval is not a safety issue but is designed to maximise the impact of the vaccination programme, as advised by the JCVI.
What should I do if I’ve already had my 1st dose?
You should plan to attend your second appointment. You should have a record card with your next appointment, which should be between 3 and 12 weeks later. Keep your record card safe and make sure you keep your next appointment to get your second dose. It is important to have both doses of the same vaccine to give you the best protection.
If the date of your 2nd dose has changed, the NHS will contact you about when you’ll have your 2nd dose.
I am due to have my 1st dose soon. When will my 2nd dose take place?
If you are due to have your 1st dose after 30 December, you’ll be given your 2nd dose around 12 weeks later.
After you’ve had the vaccine will you still need to follow all the infection control advice?
The vaccine cannot give you the COVID-19 infection. After the first dose your chance of becoming seriously ill is reduced. No vaccine is completely effective and it will take a few weeks for your body to build up protection. So, you will still need to follow the guidance in your workplace, including wearing the correct personal protection equipment and taking part in any screening programmes.
To continue to protect yourself, your patients, your family, friends and colleagues, you should follow the general advice at work, at home and when you are out and about:
- practice social distancing
- wear a face mask
- wash your hands carefully and frequently
- follow the current guidance.
The NHS is currently offering the COVID-19 vaccine to people most at risk from coronavirus.
In England, the vaccine is being offered in some hospitals – including Walsall Manor Hospital – at pharmacies, at hundreds of local vaccination centres run by GPs and at larger vaccination centres.
The vaccine was made available from Tuesday 8 December 2020 at Walsall Manor Hospital.
The order in which people will be offered the vaccine is based on advice from the Joint Committee on Vaccination and Immunisation (JCVI).
The hospital will let you know when it’s your turn to have the vaccine. It’s important not to contact us for vaccination before then.
Vaccine safety
The vaccine is safe and effective. It gives you the best protection against coronavirus.
Any coronavirus vaccine that is approved must go through all the clinical trials and safety checks all other licensed medicines go through. The UK has some of the highest safety standards in the world.
Vaccines will only be used if they are approved by the MHRA. The MHRA has been monitoring every stage of coronavirus vaccine development.
So far, thousands of people have been given a COVID-19 vaccine and reports of serious side effects, such as allergic reactions, have been very rare. No long-term complications have been reported.
Who will get the vaccine?
The NHS is currently offering the COVID-19 vaccine to people most at risk from coronavirus.
At this time, the vaccine is being offered in some hospitals – including Walsall Manor Hospital – to:
- some people aged 80 and over who already have a hospital appointment in the next few weeks
- people who work in care homes
- health care workers at high risk
The vaccine will be offered more widely as soon as possible.
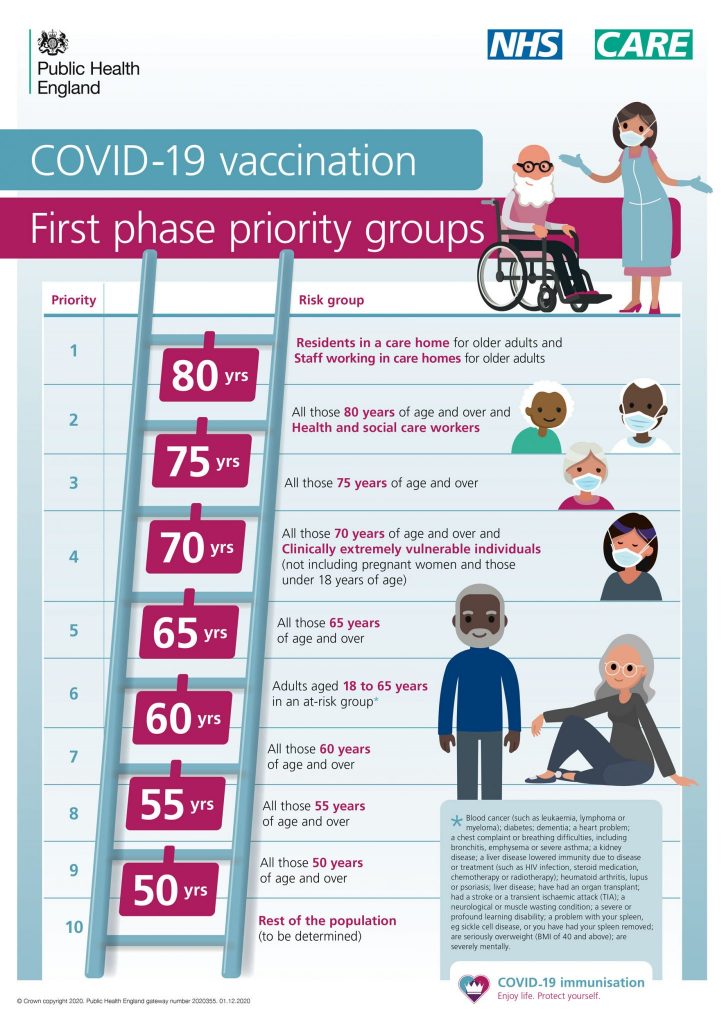
Public Health England (PHE) have published the following overview of the first phase priority groups for the COVID-19 vaccination:
The order in which people will be offered the vaccine is based on advice from the Joint Committee on Vaccination and Immunisation (JCVI).
Read the latest JCVI advice on priority groups for the COVID-19 vaccination on GOV.UK
For more information on the vaccine, visit www.nhs.uk/CovidVaccine